Phenylephrine Complex Prescribing Information
Package insert / product label
Generic name: phenylephrine hydrochloride, dextromethorphan hydrobromide and brompheniramine maleate
Dosage form: oral liquid
Drug class: Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Phenylephrine Complex Description
Phenylephrine Complex Liquid is a strawberry-flavored, clear liquid available for oral administration. Each teaspoonful (5 mL) contains:
| Phenylephrine HCl | 7.5 mg |
| Brompheniramine Maleate | 4 mg |
| Dextromethorphan HBr | 15 mg |
INACTIVE INGREDIENTS: Sodium Benzoate, Citric Acid, Saccharin Sodium, Sorbitol Solution, Glycerin, Strawberry Flavor, Purified Water.
Phenylephrine HCl is a nasal decongestant with the chemical name Benzenemethanol, 3-hydroxy-α-[(methylamino)methyl]-, hydrochloride (R)-. Its structure is as follows:
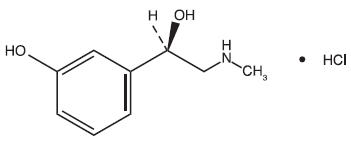
C9H13NO2•HCl M.W. 203.67
Brompheniramine Maleate is an antihistamine with the chemical name: 2-Pyridinepropanamine, γ-(4-bromophenyl)-N,N-dimethyl-, (±)-, (Z)-2-butenedioate (1:1). Its structure is as follows:
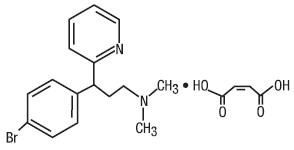
C16H19BrN2•C4H4O4 M.W. 435.31
Dextromethorphan Hydrobromide is an antitussive with the chemical name 3-Methoxy-17- methyl-9α, 13α, 14α –morphinan hydrobromide monohydrate. Its structure is as follows:
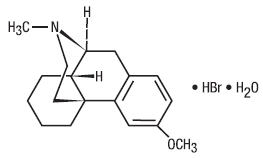
C18H25NO•HBr•H2O M.W. 370.32
Related/similar drugs
Bromfed DM, brompheniramine / dextromethorphan / pseudoephedrine, Mucinex D, Vicks Dayquil Cold & Flu Relief, Promethazine VC with Codeine
Phenylephrine Complex - Clinical Pharmacology
Phenylephrine HCl
Phenylephrine HCl is a sympathomimetic amine which acts predominantly by a direct action on alpha (α) adrenergic receptors. In therapeutic doses, the drug has no significant stimulant effect on the beta (β) adrenergic receptors of the heart. Clinically, phenylephrine shrinks swollen mucous membranes, reduces tissue hyperemia, edema, and nasal congestion; and increases nasal airway patency. In therapeutic doses the drug causes little, if any, central nervous system (CNS) stimulation.
Brompheniramine Maleate
Brompheniramine Maleate is an alkylamine-type antihistamine and is among the most potent H1 blockers, and are generally effective in relatively low doses. Although brompheniramine is not so prone as other types of antihistamines to cause drowsiness, a significant proportion of patients do experience this effect. CNS stimulation is more common with alkylamines.
Indications and Usage for Phenylephrine Complex
Phenylephrine Complex Liquid is indicated for temporarily relieving symptoms of upper respiratory tract disorders such as nasal congestion, vasomotor rhinitis and hay fever; as well as for the temporary relief of coughs associated with respiratory tract infections and related conditions such as pharyngitis, bronchitis, and asthma, when these conditions are complicated by tenacious mucus and/or mucus plugs and congestion. Phenylephrine Complex Liquid is effective in a productive as well as a nonproductive cough, but is of particular value in a dry, nonproductive cough which tends to injure the mucous membrane of the air passages.
Contraindications
Phenylephrine Complex Liquid is contraindicated in infants and newborns, and in patients with a known hypersensitivity to any of the components. It is also contraindicated in patients with severe hypertension, severe coronary artery disease, hyperthyroidism, and in patients on MAO inhibitor therapy (or for 14 days after stopping MAOI therapy). Patient idiosyncrasy to adrenergic agents may be manifested by insomnia, dizziness, weakness, tremor, or arrhythmias. Patients known to be hypersensitive to other sympathomimetic amines may exhibit cross sensitivity with phenylephrine.
Warnings
Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occurs, discontinue use and consult a doctor. If symptoms do not improve within 7 days or are accompanied by a fever, consult a doctor. Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, or emphysema, or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor. A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
May cause excitability especially in children. Sedatives and tranquilizers may increase the drowsiness effect. Do not give this product to children who are taking sedatives or tranquilizers, without first consulting the child's doctor.
Do not take this product, unless directed by a doctor, if you have a breathing problem such as emphysema or chronic bronchitis, or if you have glaucoma or difficulty in urination due to enlargement of the prostate gland. May cause drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor. Use caution when driving a motor vehicle or operating machinery.
If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, or health-care provider before using or continuing use.
Sympathomimetic amines should be used judiciously and sparingly in patients with hypertension, ischemic heart disease, diabetes mellitus, increased intraocular pressure, hyperthyroidism, or prostatic hypertrophy. Sympathomimetics may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension. Do not exceed recommended dosage. Discontinue use if adverse reactions occur. Hypertensive crises can occur with concurrent use of phenylephrine and MAO inhibitors (and for 14 days after stopping MAO inhibitor therapy), indomethacin, or with beta-blockers and methyldopa. If a hypertensive crisis occurs, these drugs should be discontinued immediately and therapy to lower blood pressure should be instituted. Fever should be managed by means of external cooling.
Precautions
Information for Patients
Use caution when driving a motor vehicle or operating machinery.
Stimulants, such as phenylephrine, are banned and tested for by the U.S. Olympic Committee (USOC) and the National Collegiate Athletic Association (NCAA).
General
Use with caution in patients with diabetes, hypertension, or cardiovascular disease. Before prescribing medication to suppress or modify cough, it is important to ascertain the underlying cause of the cough, that modification of cough does not increase the risk of clinical or physiological complications, and that appropriate therapy for the primary disease is instituted. Antihistamines have an atropine-like action and therefore should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure, hypothyroidism, cardiovascular disease and hypertension.
Drug Interactions
Beta-adrenergic blockers and MAO inhibitors (or for 14 days after stopping MAOI therapy) may potentiate the pressor effect of phenylephrine. Concurrent use of digitalis glycosides may increase the possibility of cardiac arrhythmias. Sympathomimetics may reduce the hypotensive effects of guanethidine, mecamylamine, methyldopa, reserpine and veratrum alkaloids. Concurrent use of tricyclic antidepressants may antagonize the effects of phenylephrine. Use of other vasopressor drugs during halothane anesthesia may cause serious cardiac arrhythmias. Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate and well-controlled studies have been conducted to determine whether the components of this product have a potential for carcinogenesis, mutagenesis or impairment of fertility.
Nursing Mothers
Small amounts of phenylephrine are excreted in breast milk. Use of this product by nursing mothers is not recommended because of the higher than usual risk for infants from sympathomimetic amines.
Adverse Reactions/Side Effects
The most frequent adverse reactions with this combination product are: sedation; dryness of mouth, nose and throat; thickening of bronchial secretions; dizziness.
Other adverse reactions may include:
Dermatologic: Urticaria, drug rash, photosensitivity, pruritus.
Cardiovascular: Hypotension, hypertension, cardiac arrhythmias, palpitations.
CNS: Disturbed coordination, tremor, irritability, drowsiness, insomnia, visual disturbances, weakness, nervousness, convulsions, headache, euphoria, and dysphoria.
Urinary System: Urinary frequency, difficult urination.
Gastrointestinal System: Epigastric discomfort, anorexia, nausea, vomiting, diarrhea, constipation.
Respiratory System: Tightness of chest and wheezing, shortness of breath.
Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
Drug Abuse and Dependence
Central nervous system stimulants have been abused. At high doses, subjects commonly experience an elevation of mood, a sense of increased energy and alertness, and decreased appetite. Some individuals become anxious, irritable, and loquacious. In addition to the marked euphoria, the user experiences a sense of markedly enhanced physical strength and mental capacity. With continued use, tolerance develops, the user increases the dose, and toxic signs and symptoms appear. Depression may follow rapid withdrawal.
There is no information to indicate that abuse or dependency occurs with guaifenesin or with the combination of guaifenesin, dextromethorphan and phenylephrine.
Overdosage
This product is comprised of three pharmacologically different components. Therefore, it is difficult to predict the exact manifestation of symptoms in a given individual. A description of symptoms which are likely to appear after ingestion of an excess of the individual components follows:
Signs and Symptoms
Dextromethorphan in toxic doses will cause drowsiness, ataxia, nystagmus, opisthotonos and convulsive seizures. Overdosage with sympathomimetic amines can cause cardiac arrhythmias, cerebral hemorrhage and pulmonary edema. It can also cause palpitation, tremor, dizziness, vomiting, fear, labored breathing, headache, dryness of mouth, pallor, weakness, panic, anxiety, confusion, hallucinations, and delirium.
Treatment
Treatment of acute overdosage would probably be based upon treating the patient for the symptoms of overdosage of phenylephrine as follows: The treatment of overdosage should provide symptomatic and supportive care. If the amount ingested is considered dangerous or excessive, induce vomiting with ipecac syrup unless the patient is convulsing, comatose, or has lost the gag reflex, in which case perform gastric lavage using a large bore tube. If indicated, follow with activated charcoal and a saline cathartic.
Phenylephrine Complex Dosage and Administration
Adults and Children 12 years of age or older: 1 teaspoonful every 6 hours.
Do not exceed 6 teaspoonfuls in a 24-hour period.
Children 6 to 12 years of age: ½ teaspoonful every 6 hours.
Do not exceed 3 teaspoonfuls in a 24-hour period.
This product is not indicated for use in children under 6 years of age. (see PRECAUTIONS, Pediatric Use.)
How is Phenylephrine Complex supplied
Phenylephrine Complex Liquid is a clear, strawberry-flavored, sugar-free, alcohol-free, dye-free liquid supplied in 16 fl. oz. bottles, NDC 51991-660-16.
Dispense in a tight, light-resistant container, with a child-resistant closure as defined in the USP/NF.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP Controlled Room Temperature. Protect from freezing.
Warning
Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Rx Only
Manufactured by: Tri-Med Laboratories, Inc., Somerset, NJ 08873
Distributed by: Breckenridge Pharmaceutical, Inc., Boca Raton, FL 33487
Iss. 8/09
PRINCIPAL DISPLAY PANEL - 473 mL Bottle
Breckenridge
Pharmaceutical, Inc.
NDC 51991-660-16
Phenylephrine
Complex Liquid
Antihistamine/Decongestant/
Antitussive
- Sugar Free
- Alcohol Free
- Dye Free
Description: Each 5 mL (one teaspoonful) for oral
administration contains:
Phenylephrine HCl.....7.5 mg
Brompheniramine Maleate .....4 mg
Dextromethorphan HBr.....15 mg
INACTIVE INGREDIENTS: Sodium Benzoate,
Citric Acid, Saccharin Sodium, Sorbitol Solution,
Glycerin, Strawberry Flavor, Purified Water.
Rx Only
Net Contents:
16 fl. oz. (One Pint) 473 mL

| PHENYLEPHRINE COMPLEX
phenylephrine hydrochloride, dextromethorphan hydrobromide and brompheniramine maleate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Trimed | 182050567 | MANUFACTURE | |
More about brompheniramine / dextromethorphan / phenylephrine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (7)
- Side effects
- Dosage information
- During pregnancy
- Drug class: upper respiratory combinations
